
The Beginning
The Foundation for Medical Research, Mumbai
The Foundation for Medical Research is an institution devoted to advanced laboratory research in the field of neurology, immunology and microbiology. It was established in 1975 as a Public Charitable Trust in a building provided by the Sheth family to perpetuate the memory of the late Dr. Kantilal Sheth who had built it as a private surgical hospital. The Board of Trustees included two members of the Sheth family, two members of the Godrej family, Dr. N. H. Antia and since 1982, Dr. P. R. Mahadevan.
The decision to dedicate this Foundation to the laboratory aspects of research in leprosy was a result of fortuitous circumstances. Bombay with its 100,000 leprosy patients presented ample clinical material for investigation; and it was also the largest centre for scientific research in the developing world. The Director, Dr. N. H. Antia, then chief of the Tata Department of Plastic Surgery at the J. J. Group of Hospitals, had been working in the field of leprosy since 1958 and had established a laboratory in the sixties at the Post Graduate laboratories of the J. J. Hospital for the study of nerve disease.
Commencing with meagre resources, the Foundation was fortunate in not only getting permanent recognition from the University of Bombay for the training of Post Graduate and Doctoral Students, but also obtaining Income Tax Exemption under Section 35(1)(ii) and Section 35(2A) (i.e. 100 % & 133% tax exemption respectively). The permanent recognition by the University of Mumbai for Ph.D degree in Applied Biology, Microbiology, Biochemistry enabled the recruitment of many talented students, some of whom became the Core scientists for the Institute.
The exemptions enabled the securing of substantial donations for the equipment and development of the institution from various individuals and organizations, predominantly from the Godrej and Sheth families. In 1982, the floor space was increased from 9,630 sq. ft. to 16,762 sq. ft. by the addition of 1.5 floors to the original building. In addition, major equipment worth over Rs.1 crore was obtained

- Going Ahead Phase 1
- Consolidating Phase 2 (1998 – 2006)
- Expansion Phase 3 (2007 – 2015)
- Sustaining Phase 4 (2016 – 2022)
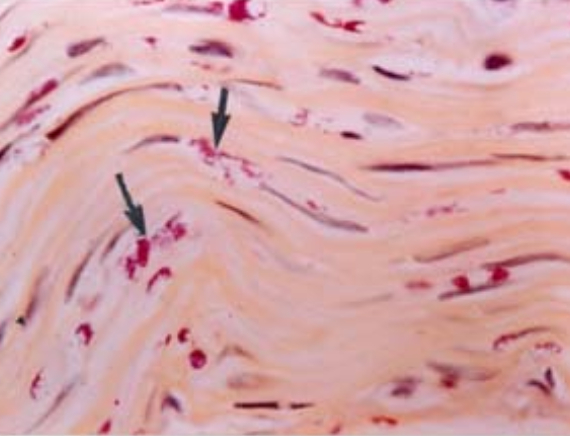
Going Ahead Phase 1
In the first fifteen years, the Foundation concentrated on focused research work in leprosy encompassing:
- Identification of mechanism of nerve damage
- Identification of pathways of immunological unresponsiveness
- Cultivation of M.leprae
The knowledge was applied to:
- Prevention and treatment of deformities in leprosy
- Development of screening assays for conventional and potential anti-leprosy drugs.
- Critiquing and developing, preventive (vaccines) and immunotherapeutic approaches to leprosy treatment and devising of immunodiagnostic tests.
The work during this period was supported entirely through donations from the Trustees though funding through Project grants was initiated in the late 80s.
In the late 90s – the FMR diversified gradually into the fields of tuberculosis and medicinal plant research. The focus in tuberculosis was drug resistance, a choice only recently vindicated by the recognition of its worldwide threat. In medicinal plants, pre-clinical screening was adopted as the nodal point due to an almost complete absence of this discipline in India for infectious diseases. Work in both areas was undertaken in the laboratory as well as in the field and a strong networking was done with reputed national and international groups. Several joint projects in the field were undertaken with the sister FRCH. This provided a strong public health perspective to the work and also resulted in the conceptualization of new approaches – both mechanisms based as well as in control and operation programmes.

Consolidating Phase 2
In 1998, the Foundation leased ~ 3,700 sq.ft of its ground floor premises to a Bank to meet its recurring infrastructure and administrative and a part of the scientific expenditure.
Whereas donations from donor Trustees are forthcoming, the bulk of the funding is now drawn from project grants. Salaries of research staff, lab supplies and materials and equipment was drawn substantially from these grants. Recourse to Core funds for scientific expenditure is during interim periods between Projects and salaries of senior scientists whose salaries are not usually met by Project funding. Over Rs.11 crores has been secured in these years as Project funds. In 2007 funds were augmented through the award of a Corpus Grant from Jamsetji Tata Trust for an amount of Rs. 60 Million (Rs. 6 Crores). The Corpus Grant was awarded for the purpose of meeting “institutional Core costs for stability and autonomy of the organization to enable them to carry on the diversification and expansion of its research activities”.
The Corpus funds over the last 6 years have ultimately resulted in the following benefits:
- Contributed to the financial and staff stability at FMR
- Allowed extensive networking with experts and institutions for the strengthening and dissemination of work
- Enabled the undertaking of seed projects and projects refractory to securing of external funding
- Expanded capacity in addressing concerns in public health

Expansion Phase 3
Since 2007, the work at the Foundation has veered from the sole undertaking of experimental studies to community level studies at least in the fields of leprosy and tuberculosis.
With the support of the Corpus funds augmented by the license fees received from the Bank, the Foundation has been able to undertake translational activities based on the findings of basic research (viz. active surveys, infection control measures, advocacy).
The FMR has expanded its repertoire of Public Health skills in the fields of visual epidemiology, qualitative research and contemporary data collection technologies. Additionally it was able to include the discipline of data management and basic epidemiology as a part of available cross cutting expertise for its researchers.
There has been a significant strengthening of expertise and technology in molecular biology for both TB and leprosy. This extends from molecular epidemiology to molecular phylogeny and currently whole genome sequencing. The staff and students of the Foundation have now a comfortable access to training and skill building opportunities.
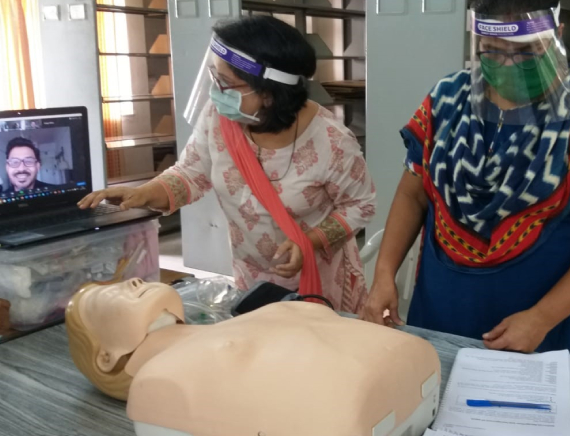
Sustaining Phase 4
This period has witnessed the entry of large-scale genomics and transcriptomics projects in its repertoire in TB with the dual objectives of understanding anti-microbial resistance and TB transmission.
An innovative non-invasive mask-based sample collection method was standardized to compute pathogen emission. These years also witnessed an invitation from Gates Foundation to study private sector engagement in TB control in Mumbai and Patna.
The modernization of plant-based medicine focussing on the development of the guava leaf extract as an anti-diarrhoeal passed the 2 benchmarks of an efficacy clinical trial and subsequently the standardization of the guava leaf extract (GLE) through the use of the modern technology of metabolomics. This phase also witnessed the entry of environmental research in water bodies in Maharashtra where the themes of pollution, community resilience and horizontal transmission of anti-microbial resistance were probed into.
Research in leprosy dealt with the prevention of nerve damage through clinical trials with high dose steroids and bringing to light challenges to patients with continual post-treatment deformities.
Two areas of research were added on to the FMR project cassette in this phase. Health systems strengthening included devising of in-service approaches for strengthening capacities in maternal health of a critical front-line cadre, the auxiliary nurse midwife. The development of a cadre of competent public system master trainers for in service training was a key objective.
With the onset of the COVID-19 pandemic in March 2020, the Foundation could rapidly mobilize its public health expertise in understanding transmission kinetics of SARS-CoV2 viral variants during pre and post-vaccination stages. Environmental surveillance of viral variants in waste water has been currently initiated.
A key hallmark of this phase were strong attempts by FMR to upskill the public health system in the fields of whole-genome sequencing for tuberculosis and maternal health with a focus on competent handling of obstetric emergencies. The incorporation of modern technologies such as WGS, RNA sequencing, metabolomics and detection of drug-resistant elements in bacterial genomes were a fruition of wide networking with private physicians, public and private facilities in India as also international academic institutions.
A biosafety level-3 facility was built through corporate and private donations to undertake work on high-risk pathogens (TB, COVID-19) with safety. This facility is certified by the Department of Biotechnology. In line with regulations, FMR instituted an Institutional Biosafety Committee under the oversight of the Department of Biotechnology.
A new Research Advisory Board was instituted with distinguished members in 2021 to guide both financial and academic management at the Foundation.
In line with the vision of our founder, Dr. Noshir Antia, the process of convergence of the Foundation for Medical Research with its sister Foundation for Research in Community Health (FRCH), Pune has been initiated to bring about work cohesion through a duality of the medical and the social sciences. This emphasizes the importance of both medical and non-medical determinants of health and disease.
